Summary Results of Placebo Controlled Long-term Activated Air Therapy Study of Chronic Obstructive Pulmonary Disease (COPD) Patients
Study conducted by Dr. Michael Kucera
This study was undertaken to evaluate the use of inhaled Activated Air as a therapy for supplementing existing treatments for COPD.
A total of 24 patients were studied over a six-month period. Half the patients were treated with a placebo and half were treated with activated air. Patients treated with activated air showed significant improvement while the placebo group showed no improvement. These data suggest that activated air has significant potential for COPD treatment.
Introduction
Chronic Obstructive Pulmonary Disease (COPD) is a progressive disease resulting in reduction of pulmonary functions that can lead to respiratory failure and cardiovascular complications. The prevalence of COPD is 8-10% with relatively high mortality (around 25% for male patients, around 14% for female patients). Therapy for the disease is very complex and should be continuous although it can worsen the patients’ quality of life.
The goal of this study was to assess the possibility of inhaled activated air as a supplemental therapeutic method to existing treatment for COPD.
Methodology and Material
A placebo controlled study started in March 2008 and ended in March 2010. The study was done on two groups of 12 male patients, as described below.
Group 1 – Verum:
- 4 smokers
- 8 non smokers
- age ranged from 49 to 67 years, average 58,08±5,08
- treatment was two 20-minute sessions each day for 6 months using Active Air therapy modus AU 5/5
- Patients had been diagnosed with COPD between 4 and 10 years
- Spirometry parameters FEV1 % and FEV1/FVC % were measured at the beginning and end of the study
Group 2 – Placebo:
- 4 smokers
- 8 non smokers
- age ranged from 49 to 66 years, average 57,45±5,77
- the inside of the activate air device was modified using a shunt. The device operated normally but the patient did not know that he was inhaling normal air that had not passed through the activation chambers. All other aspects of use were the same with two 20-minute sessions each day for 6 months using Active Air therapy modus AU 5/5
- Patients had been diagnosed with COPD between 5 and 11 years
- Spirometry parameters FEV1 % and FEV1/FVC % were measured at the beginning and end of the study
Patients were evaluated with Spirometry.[1] SPIROLAB II, produced by MIR Medical International Research was used. Statistical significance was evaluated using the Chi Square-test and Student t-test.
Spirometry Results
Spirometry measures for each patient, taken prior to starting and at the end of the treatment, are shown below. In every case the treatment group showed improvement.
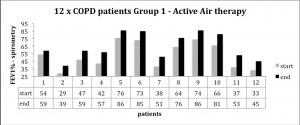
The placebo group did not show improvement during the six-month study period, as seen in the chart below.

Inhalation of activated air significantly improved spirometry parameters. The improvement was statistically significant in both monitored parameters: FEV1/FVC% and FEV1%. This improvement was followed with subjective improvement of dyspnea and expectoration and with reduced frequency of medical inhalants.
GOLD Evaluation
The Global Initiative for Chronic Obstructive Lung Disease (GOLD) is a four-stage classification of COPD severity. GOLD evaluation is based on the patient’s symptoms and the results of the lung function test, according to the guidelines below.
Gold Evaluation Guidelines
| Stratum | FEV1 / FVC | FEV1 | Symptoms |
| 1 (mild) | less than 70 % | 80 % | with / without symptoms
(cough, expectoration) |
| 2 (moderate) | less than 70% | 50-80 % | with / without chronic symptoms (shortness of breath, cough and expectoration) |
| 3 (severe) | less than 70% | 30-50 % | with / without chronic symptoms (shortness of breath, cough and expectoration) |
| 4 (extremely severe) | less than 70% | less than 30 % or less than 50 % and chronic respiratory failure | quality of life noticeably impaired, exacerbation can be life threatening |
This study considered the change in the GOLD classification for both the Verum and placebo groups. Results of the GOLD evaluation for the group using activated air were as follows:
- 1 patient from stratum 4 to stratum 3
- 3 patients from stratum 3 to stratum 2
- 4 patients from stratum 2 to stratum 1
- 2 patients improvement within stratum 3
- from FEV1 38 to 51 – from FEV 1 /FVC 59 to 66
- from FEV1 33 to 45 – from FEV 1 /FVC 60 to 68
- 2 patients improvement within stratum 2
- from FEV1 54 to 59 – from FEV 1 /FVC 61 to 64
- from v 64 to 76 – from FEV 1 /FVC 60 to 67
Summary
The results show, that Active Air treatment for 6 months 2 times daily (home use) significantly improve the spirometry parameters. In short the study group using activated air showed improvement not found in the placebo group:
- Significant improvement in both parameters FEV1 % und FEV1/FVC %
- Subjective improvement the dyspnoea
- Improvement in Expectoration
- Less inhalation medicine
- Significant overall improvement based on the GOLD classification standard
Forced Vital Capacity (FVC) is the volume of air that can forcibly be blown out after full inspiration, measured in liters. Forced Expiratory Volume in 1 Second (FEV1) is the maximum volume of air that can forcibly blow out in the first second during the FVC maneuver, measured in liters. Along with FVC it is considered one of the primary indicators of lung function. The FEV1/FVC ratio is a calculated ratio used in the diagnosis of obstructive and restrictive lung disease. It represents the proportion of the forced vital capacity exhaled in the first second. Normal values are approximately 80%.